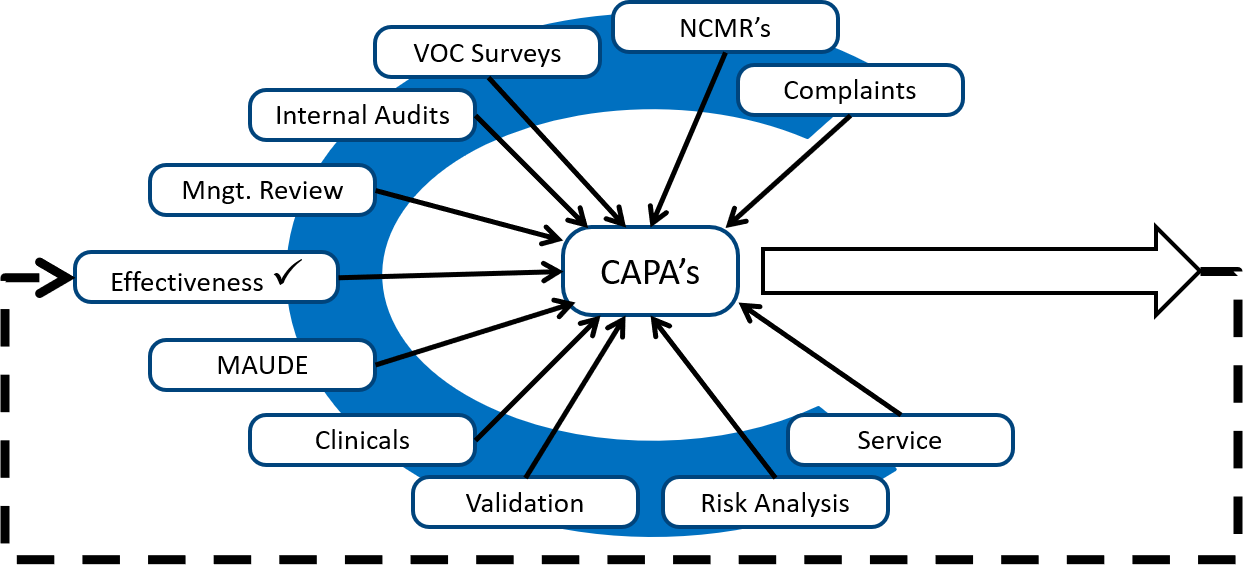
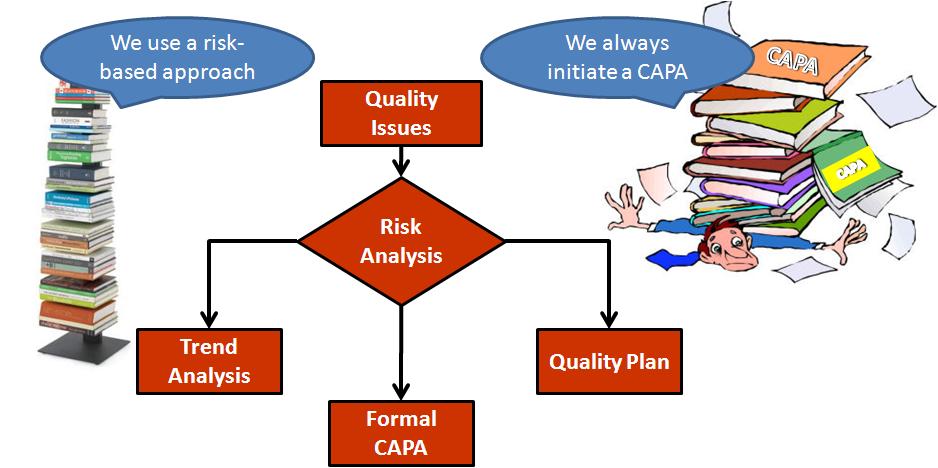
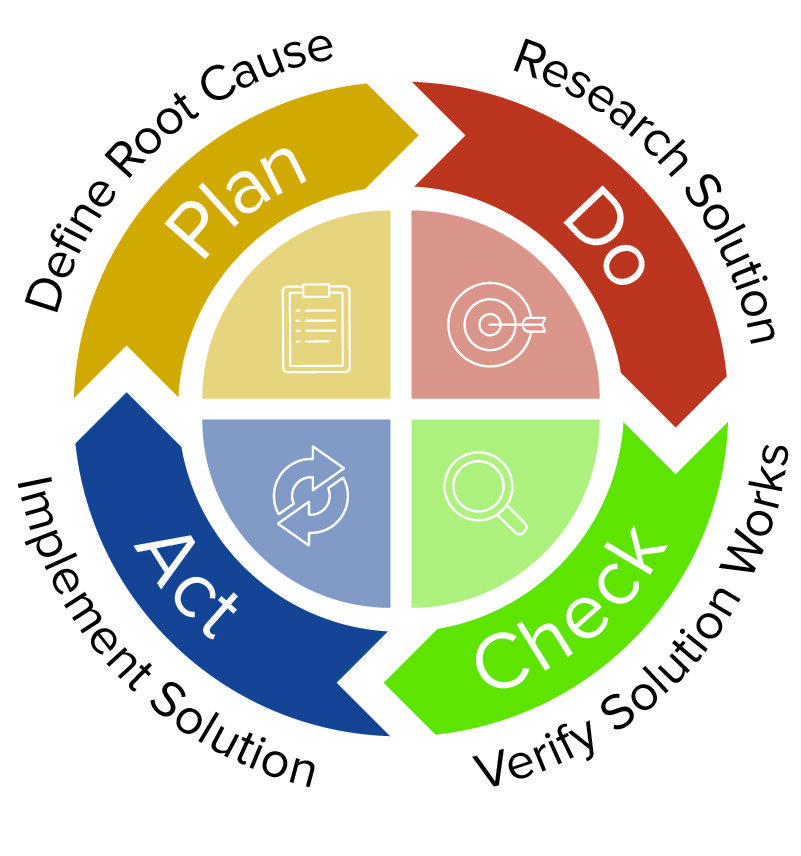
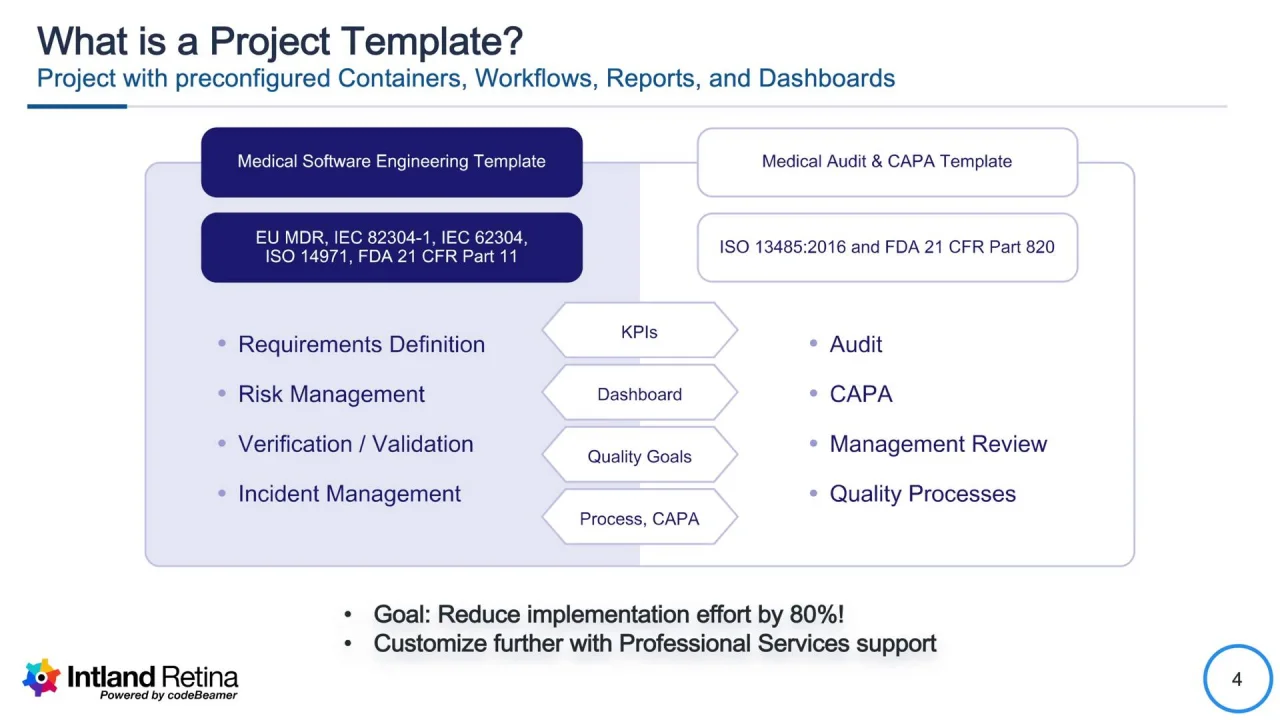
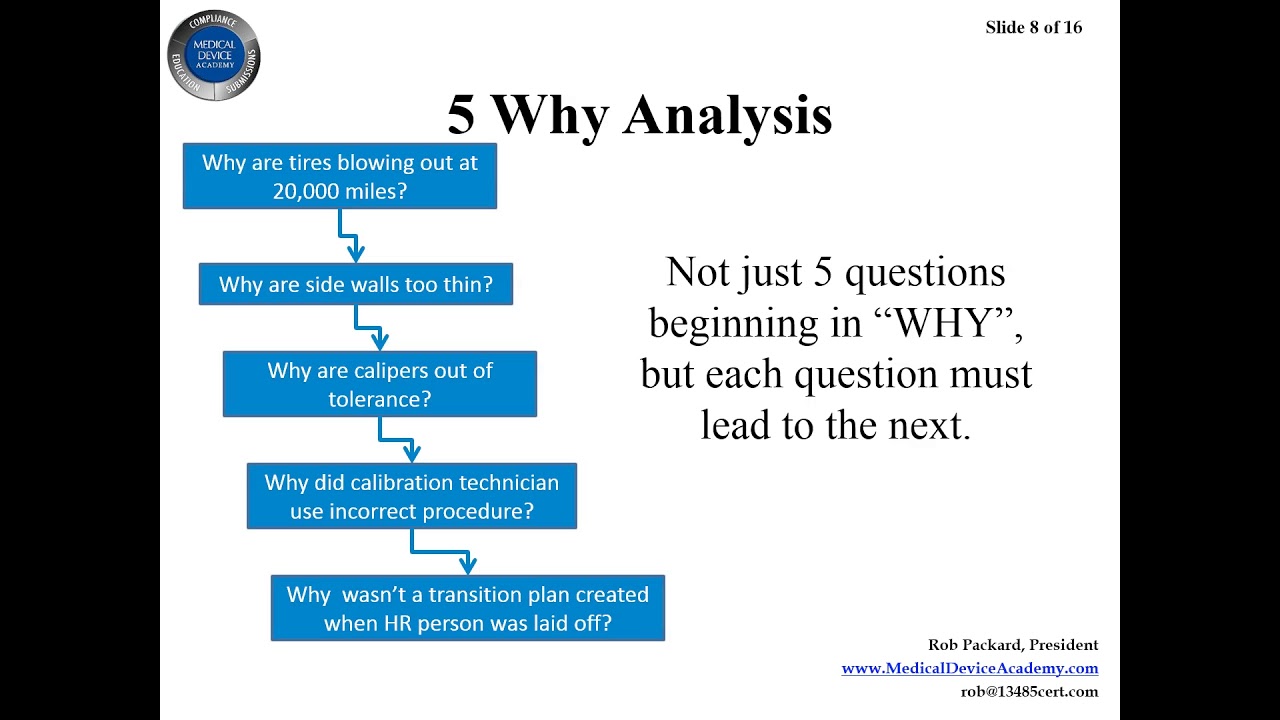
Medical Device CAPA Process : Importance, benefits and implementation | Engineering - Johari Digital Healthcare Ltd.

CAPA Certification Assistance | Qualitas Compliance Raleigh | Medical Device Compliance Consulting, ISO 13485, Durham, Chapel Hill, RTP, Boston, Charlotte, Atlanta, Pittsburgh, Tampa, medical device risk management, fda submissions, gap analysis, CAPA